Gilead: Cultivating the Pipeline
By: Jason Cheung & Dennis Zhan
The Ivey Business Review is a student publication conceived, designed and managed by Honors Business Administration students at the Ivey Business School.
Symptoms
Just a couple years ago, Gilead Sciences was a different company than it currently is, but not for the better. Gilead specializes in the production of a high-performance drug that counteracts the infectious hepatitis C (HCV) disease as well as biologically similar ailments. While still one of the largest biopharmaceutical companies in the world that discovers, develops and commercializes therapeutics, Gilead faces structural challenges in the segments it operates in, specifically in HCV. Despite their patent lasting until 2028, revenue and earnings are facing compression from HCV dependence amidst declining HCV market growth and slowing demand. As a reflection, Gilead has experienced a stark stock price decline that seems unlikely to cease, trading down to $73share as of February 2, 2017 from its high of $122 in June 2015. In order to restore investors faith and tackle its core business problems, Gilead needs to diversify into other channels of sustainable revenue generation to minimize revenue volatility.
Core Business Problems
Historically, the sale of HCV products has accounted for roughly 56 per cent of Gilead’s revenue since 2014. Looking forward, investors question Gilead’s reliance on HCV treatment demand as supply is becoming saturated; AbbVie, Bristol-Myers Squibb, Johnson & Johnson, and Merck have all developed substitute offerings in this segment. The transition of market dynamics induced pricing reductions; Gilead currently sells HCV products at significant discounts to their list prices.
In its latest quarterly report, Gilead estimates 2017 full-year revenue to be between $22.5-billion to $24.5-billion. A figure well short of analyst estimates of $28-billion, this is an artefact of decreased domestic sales and international expansion shortcomings. Additionally, Gilead has less in their developmental pipeline, the main predictor of future revenues, in comparison to competitors such as GlaxoSmithKline and Pfizer. The strategic implication for Gilead is that it needs to increase drug discovery to remain competitive in the long run. However, internal research and development (R&D) are not a strength of Gilead nor large-cap pharmaceuticals in general. Studies indicate that smaller biotech groups produced an average 17 per cent return on R&D investment over the past three years, compared with five per cent for twelve big groups. Faced with weak returns on development ventures, Gilead has favoured returning cash to investors through large dividend payouts and stock buybacks.
Core Competency
The strongest bull argument for Gilead lies in its ability to generate substantial cash. Gilead’s core competency is producing drugs with higher margins than their competitors. In the last four years, Gilead has grown their gross margin from 75 percent to 88 per cent and their operating margin from 41 per cent to 68 per cent. Gilead is sitting on a cash balance of $12-billion and has produced a trailing operating cash flow of $15.3-billion. However, the longevity of healthcare companies depends on identifying alternative growth ventures. Consider the life cycle of drug products from the perspective of a developer. Upon approval from regulatory bodies, supply management controls effectively create a monopoly during an exclusive rights period before the segment is opened up to generics. Portfolio turnover is a common occurrence, signalling that cash generation efficacy must be coupled with constant innovation and efficient resource allocation.
Historically, Gilead has pushed their performance to new heights with mergers and acquisitions (M&A). Gilead executed a substantial biotech value acquisition, the purchase of Pharmasset in 2011 at an 89 per cent premium for $11-billion. After an exhaustive analysis of Pharmasset’s drug pipeline, Gilead identified two potential crown jewels: Harvoni and Sovaldi. Today, these two drugs form the foundation of Gilead’s HCV franchise that has propped up their aggregate revenue and added more than $100-billion to their market capitalization. Other notable transactions include the purchases of Myogen and CV Therapeutics for $2.5-billion and $1.4-billion, respectively. Over the past five years, Gilead’s proven success in acquisition integration is reflected by average return on invested capital (ROIC) of 35.7 per cent. For context, Pfizer’s average ROIC was 11.2 per cent over the same time frame.
New Venture Attractiveness
An emerging market that Gilead has yet to establish a footprint in is medical marijuana (MMJ). Projecting 17.1 per cent compound annual growth, analysts predict that MMJ will represent a $55.8-billion industry by 2025. A multitude of positive regulatory developments in 2016, including 29 U.S. states legalizing the intake of marijuana for medical uses, suggest a favourable industry outlook. The Canadian market is also attractive as MMJ use is already fully legalized; it serves as the North American hub for MMJ production, regulation, and research. Lastly, a wave of progressive policies toward MMJ is sweeping across Europe. Germany is the most recent country to legalize MMJ and a plethora of European countries are looking to follow suit.
Effective commercialization of a therapeutic treatment presents pharmaceutical companies with recurring revenue opportunities. Patients require a consistent supply of MMJ in order to effectively manage their symptoms of chronic diseases such as arthritis, epilepsy, and post-traumatic stress disorder. Therefore, the MMJ market becomes a more attractive investment for Gilead as opposed to drugs that solely treat acute diseases such as HCV. While there has been an influx of producers looking to capitalize on market trends, pharmaceutical companies have been slow to react. Only 26 per cent of current research in the industry is being conducted by biotechnology companies and a minuscule two per cent by global pharmaceuticals. Currently, only two cannabinoid products, Cesamet and Marinol, have been approved by the Food and Drug Administration (FDA) in the North American market. The recent changes in awareness and regulation have primed the MMJ industry for massive potential growth. Yet, the sluggish response from pharmaceuticals leaves room for Gilead to be the first large-cap player to gain patents and reap the rewards. To properly tap into the MMJ sector and address future revenue concerns, Gilead should acquire specialized MMJ pharmaceuticals.
Spotlighting GW Pharmaceuticals
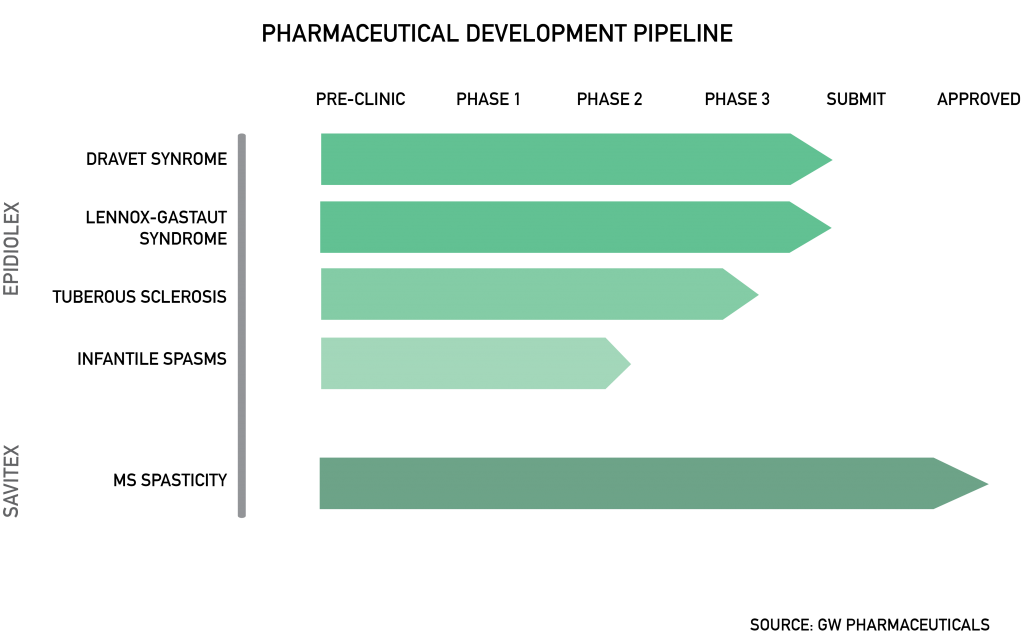
Based out of London, United Kingdom (UK), GW Pharmaceuticals is the leading cannabinoid biotechnology company in the world with respect to size and research pipeline. Their flagship product is Sativex, a cannabis-based mouth spray that has been approved for multiple sclerosis treatment in 28 countries globally. GW also has an extensive research pipeline, with their lead candidate Epidiolex in late stage clinical trials with the FDA and European Medicines Agency for Dravet Syndrome and Lennox-Gastaut Syndrome. Both agencies have granted Epidiolex an orphan drug designation that provides a seven-year exclusivity period in the United States (US) and potential tax credits for research conducted. Additionally, Epidiolex has been granted fast track approval by the FDA for the treatment of Dravet Syndrome, accelerating its route to commercialization.
Even though GW has successfully commercialized Sativex, the R&D costs associated with their pipeline greatly outweigh revenues, resulting in a net loss of $82.2-million in 2016. Cash flow from operating activities have been decreasing rapidly from a $19.9-million outflow in 2014 to a $155-million outflow in 2016. Consequently, GW has been financing operations through multiple secondary equity issuances, diluting shareholder value. As GW continues to grow and incur incremental commercialization costs, a more sustainable method of financing would be welcome. A takeover would provide much-needed cash flow and liquidity for management.
GW’s leadership should also be receptive to a letter of purchasing intent. In terms of timing, a letter of intent is usually submitted after regulatory approval of pipeline drugs. GW would be forfeiting significant upside if it engages in a sale prior to a more concrete scope of its developmental properties. Hence, it is likely that Gilead would be required to pay a well-above average premium in order to entice management and equity holders of GW. Over the last three years, the average premium paid for U.S. biopharmaceutical companies in a public takeover was 44.3 per cent. Even if Gilead was forced to pay an aggressive premium of 100 per cent, the transaction would still make sense from a perceptions standpoint as the acquisition would be a strong indicator that Gilead is conscious of its core business problems. However, this acquisition is much more than optics; it provides an entrance into a rapidly growing market that compliments Gilead’s existing operations and strengths.
Investment Proposal
From the perspective of Gilead, an acquisition of GW would be a strong prospect from a capabilities standpoint. Gilead has demonstrated the ability to commercialize drugs effectively whether through organic development or an out-licensing agreement. Gilead’s strong performance in the operational and marketing aspects of the value chain have culminated into strong margins management and industry-leading distribution performance domestically. To get a sense of their superior margins, Gilead generates a 45.1 per cent net profit margin whereas Pfizer trails drastically at 13.7 per cent. According to Bloomberg, Gilead’s HCV franchise became the top-selling new product in the history of the drug industry. These artefacts support the assertion that Gilead lacks only ideation in their business model. Gilead has yet to establish a strong franchise for neurological diseases. Additionally, Gilead’s $12-billion offshore cash balloon is in danger of being reduced by U.S. corporate tax rates. Incorporated in the UK, GW would provide an ideal opportunity for Gilead to avoid repatriation, expand their European presence, and engage a growing market. All in all, the acquisition of GW will engage a proprietary treatment methodology that has substantial traction and balances their existing portfolio, a requisite in the eyes of investors.
Additionally, to continue future advancement in the MMJ space, Gilead should also seek to acquire clinical stage entities such as Zynerba Pharmaceuticals. A specialty MMJ company, Zynerba is still in the experimental stage with their pipeline. The flagship product of Zynerba is ZYN002, a synthetic cannabidiol gel that is applied through transdermal delivery for the treatment of epilepsy, osteoarthritis and Fragile X syndrome. Under development, ZYN002 is in Phase II of the FDA process and has undergone safety and tolerability reviews at the American Epilepsy Society. By acquiring GW, Gilead adopts a guaranteed product to capitalize on the MMJ industry, whereas Zynerba allows Gilead to continually grow their pipeline and gain long-term sustainability. Pharmaceutical roll-ups are a part of the consolidation process, which occur as new market sectors mature and offer R&D cost synergies.
In the case of Gilead, investors have discounted the past success of HCV sales as an aberration and not an indicator of future dominance. It is hard to find fault in that position as the decline in product sales is occurring at an alarming rate. During the latest quarter, year-over-year sales of Harvoni more than halved to $1.6-billion, while Sovaldi sales plunged from $1.6-billion to half a billion dollars. Finding future growth opportunities is paramount for Gilead. Since the main driver for growth in the pharmaceuticals industry is M&A, it makes sense for Gilead to engage GW even if the terms are not ideal. Gilead is unlikely to deliver substantial incremental increases to its bottom line through cost-cutting. Furthermore, international drug distribution efforts are not a feasible contingency. Other potential industry obstacles such as downward pricing pressures, patent cliffs, and the threat of generics demand a call to action. Complacency would be a catastrophic position for Gilead. Consider, any move that appears to be put in good faith towards delivering future growth would be looked upon favourably by retail investors; the subsequent reversal of stock price performance would outweigh the one-time investment made by Gilead by many magnitudes.
As evidenced by more than 69 per cent of Gilead’s acquisitions closing before 2013, the company needs to prioritize an acquisitive strategy in non-traditional medicines going forward. Innovative companies target start-ups and other private entities to take advantage of promising breakthrough medicines which could significantly improve the acquirer’s attractiveness. Gilead must emphasize acquiring ideas with a strategic value that may not translate into immediate financial results. Since most industries progress predictably through a clear consolidation life cycle, Gilead should focus on executing the roll-up of middle market MMJ firms. Thankfully, the torrid times that have engulfed Gilead are pronounced enough to induce a cultural shift.